Rare Diseases
Rare diseases collectively affect 5% of the world’s population. Up to 80% of rare diseases are genetic in origin. Additionally, nearly 50% of all rare disease patients are children.
THREAD's Rare Disease Experience
.png)
Almost 60 Decentralized Rare Disease Studies, with 95% with a hybrid design and 5% fully virtual design
.png)
Experience across all drug development including Natural History Studies, Disease registries and Phases I - Phase IV, with 60% of work being done in Phase III
.png)
Across multiple therapeutic areas including: Neurology, Hematology and Oncology, Endocrine, Pulmonary, Cardiology and Dermatology
.png)
Across 40 countries

Expert Insight for Rare Disease

Challenges in Rare Disease Trials & How Decentralized Trials Can Support
The ability to implement DCT elements as a means of reducing the burden of study participation for patients and their caregivers can have a positive impact on the industry’s ability to conduct studies, especially for more challenging therapeutic areas, such as rare diseases.
How Rare Diseases Were Affected by the COVID Pandemic
A recent issue of Raconteur (published as a supplement in The Times, UK) took a deep look at the rare disease landscape, including how rare diseases were affected by the COVID pandemic, and how patients who are engaged in the drug development process through clinical trials can build connections through digital platforms.
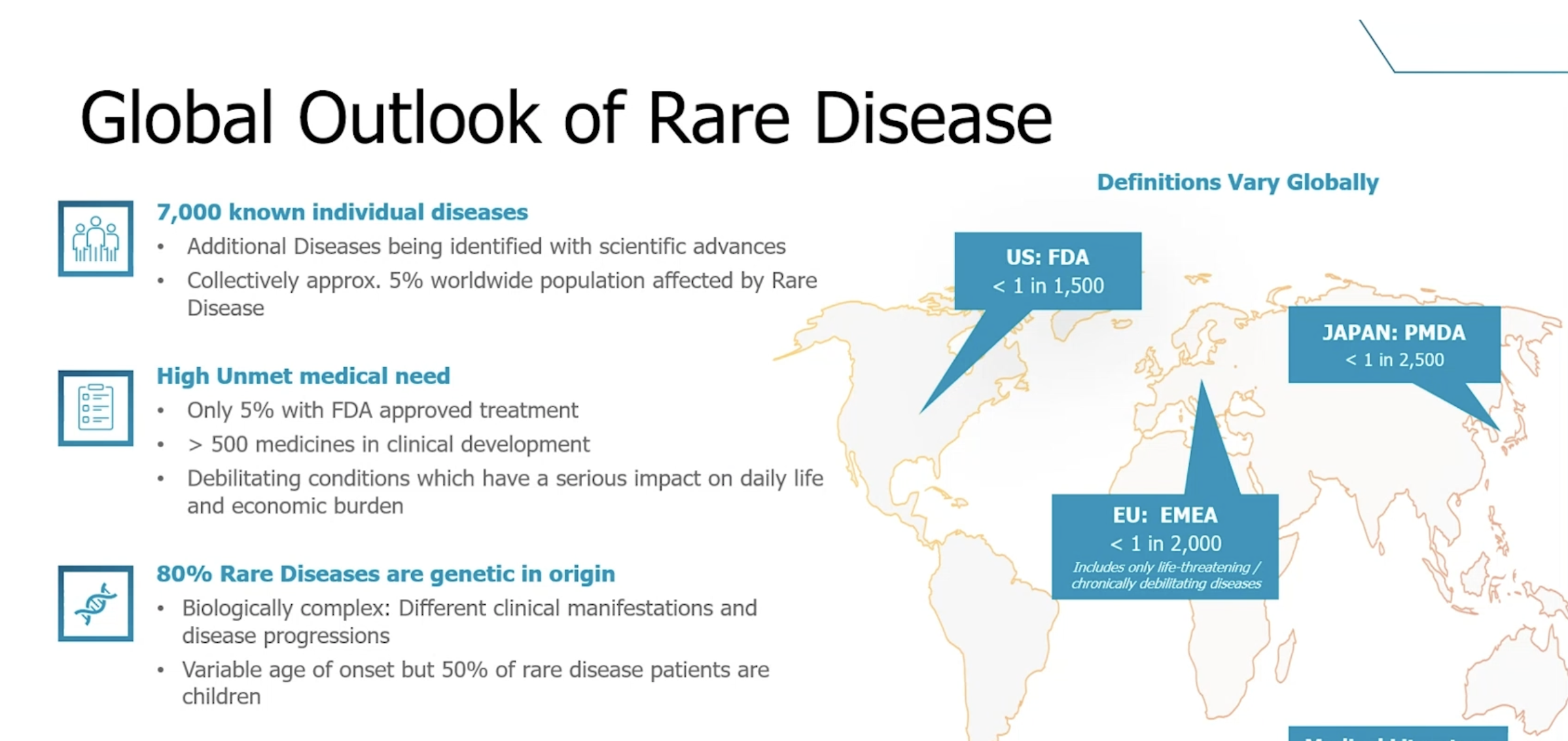
Challenges in Rare Disease Studies and How Decentralized Trials Can Help
Decentralized Clinical Trials (DCTs) provide an opportunity to transform participation in rare disease clinical trials. Moving from the traditional site-based model to DCT elements that can be brought to participants’ homes can reduce the overall study burden and improve the participant experience leading to better engagement and retention.

Pediatric Rare Diseases - By the Numbers
75% of rare diseases have exclusive pediatric onset, with 66% appearing before two years of age. Download the infographic to learn how Decentralized Clinical Trials (DCTs) can help broaden patient access to clinical research and improve the patient and family experience.

3 Tips for Applying DCT Approaches in Clinical Trials
Decentralized clinical trial (DCT) approaches can be extremely useful in helping to facilitate clinical research participation for rare disease patient populations. That said, many sponsors new to conducting studies that include DCT elements may need some help getting started. Discover THREAD’s 3 approaches that have helped sponsors execute their rare disease studies.

Audiogram
Caregivers are a crucial part of the clinical trial process. Listen to this perspective of a rare disease caregiver, as she discusses listening to the patient, her needs and the positive changes she has seen in her daughter.
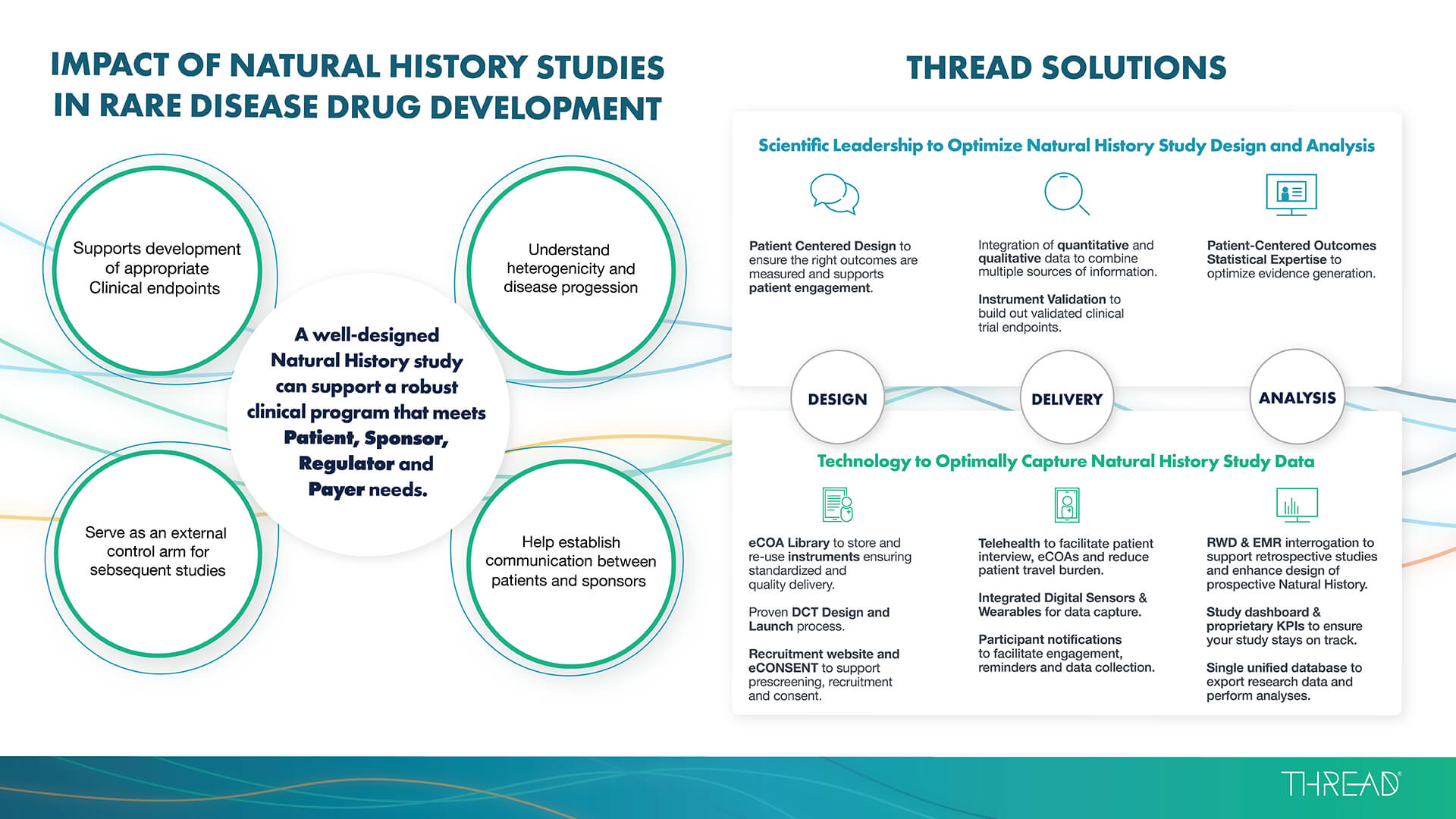
Natural History Infographic - By the Numbers
A well-designed natural history study can support a robust clinical program that meets patient, sponsor, regulator and payer needs. Download the infographic to learn how THREAD’s solutions can benefit your rare disease studies
-2.png?width=1800&height=363&name=MicrosoftTeams-image%20(18)-2.png)